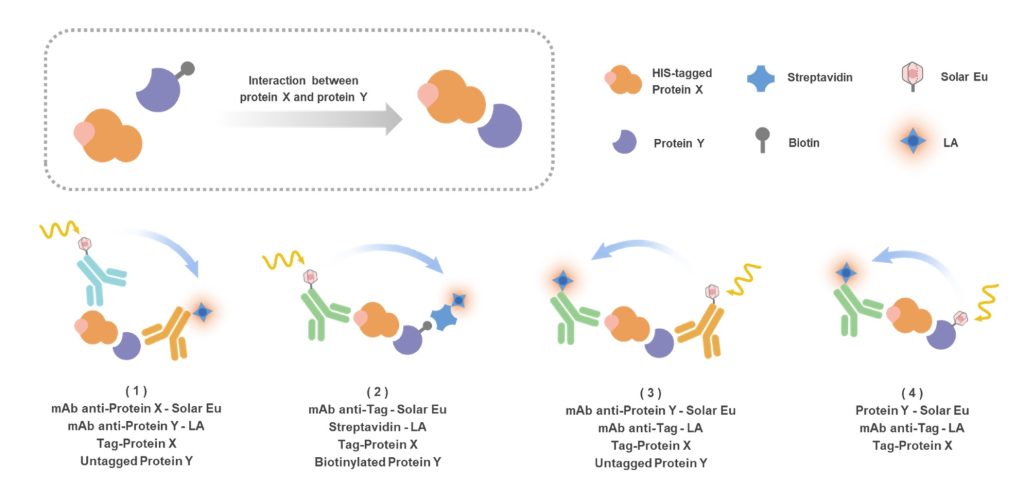
TR-FRET (Time-Resolved Fluorescence Resonance Energy Transfer) is a powerful homogeneous detection technology designed for sensitive, quantitative analysis of biomolecular interactions. By integrating classical FRET with time-resolved fluorescence detection, TR-FRET effectively suppresses background autofluorescence and delivers exceptional signal-to-noise performance—even in complex biological systems.
In a TR-FRET assay, two interacting molecules are brought into proximity through direct binding or molecular recognition. One is associated with a long-lifetime lanthanide donor (such as europium or terbium), while the other carries a compatible acceptor fluorophore. Upon excitation, energy transfer occurs only when the two labels are within nanometer distance, generating a highly specific fluorescence signal proportional to the interaction strength.
Key Advantages
- Homogeneous, mix-and-read workflow – no washing or separation steps
- Ultra-low background via time-gated detection
- High sensitivity and reproducibility
- Stable signals with flexible readout timing
- Easily scalable from 96-well assays to 384- and 1536-well HTS formats
- Excellent tolerance to DMSO, buffers, and assay additives
TR-FRET is particularly well suited for applications where accuracy, throughput, and robustness must coexist.
Applications
- Protein–protein interaction (PPI) analysis
- Receptor–ligand binding studies
- Antibody–antigen interaction assays
- Competitive binding and inhibitor screening
- High-throughput drug discovery and assay development